Educational and Reference Resources of the Tri-Canyons
Ecology in the Canyons: Snow Science & the States of Matter
Whether you’re joining us in the great outdoors, the classroom, or your own backyard, we’re excited to keep learning more about science and ecology with you.
This month we’re going to learn about snow science and the states of matter. We’ll learn about:
- Molecules & the States of Matter
- How Matter Changes States
- How Clouds Form
- How Rain & Snow Form
- How to Make Ice Cream Out of Snow
We hope that you will enjoy learning! You may use the Table of Contents to go directly to any specific topic.
Introduction: What is Snow?
Big, powdery flakes of snow are a familiar sight during most Utah winters. If you’re anything like me, you get the urge to throw a coat on and run outside the first time you see snow falling every year. Maybe you like to sled, ski, or snowboard. Maybe you enjoy making snow forts, snow people, or pelting your friends with snowballs. Most of us are pretty familiar with snow – but what is snow?
You probably just answered “Snow is frozen water!” And you’re right. Most of us understand that when you heat snow up, it melts into liquid water. But if you put that water into the freezer, it doesn’t turn back into snow, does it? It turns into ice. So how is snow made? What is the relationship between water, ice, and snow, and what’s happening in nature that is so different from our freezer?
These might seem like simple questions, but to answer them well, we need to understand some really interesting things about matter and weather. Let’s start by reviewing the different states of matter. Understanding how matter changes between a solid, a liquid, and a gas will be our first step toward appreciating how weird and wonderful snow is.
States of Matter
Our world and everything in it is made up of matter. All matter is made of tiny, microscopic particles called atoms. We have discovered 118 different kinds of atoms, which we call elements. Elements come together in combinations called molecules, and these molecules make up everything in the universe. That includes the sun, the earth, the air we breathe, and you! That was a lot of information, so let’s go over it one more time: everything in the world is made out of different molecules, which are combinations of different elements.
Molecules are microscopic, which means they are too small to be seen with our eyes. But huge numbers of molecules form substances that we can sense, meaning we can see, touch, feel, taste, and smell them. We can’t sense a single molecule of water, but when thousands of water molecules combine, we can drink them, take a bath in them, or sled down a hill covered in them.
A single molecule of water is made of three atoms: two hydrogen atoms and one oxygen atom. That’s why water has the chemical formula (or name) H2O.
So now we understand what water is, right? Water is a group of H2O molecules that is big enough for us to interact with. And like any group of molecules, water can exist in different forms. If we cool water down, it freezes into ice. If we heat water up, it evaporates into water vapor. But why does this happen? How does changing the temperature of a substance change its state? To understand the answer, we have to learn a little more about molecules and the way they behave.
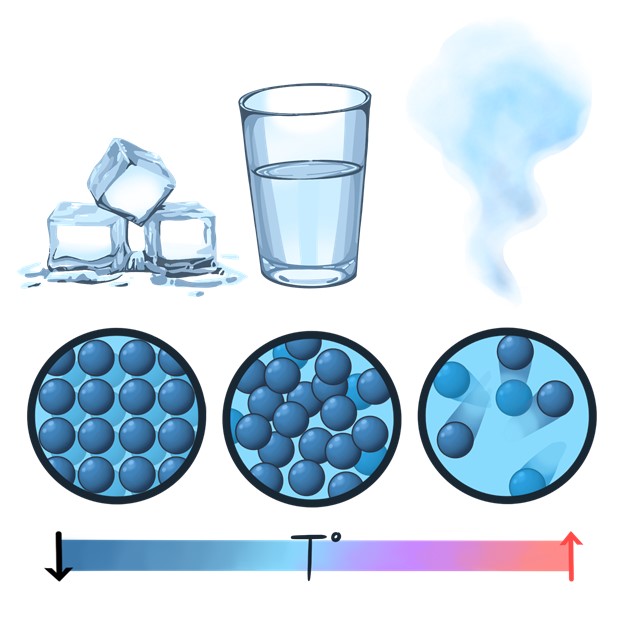
When the molecules inside a substance heat up, they gain energy and begin to move around. When the molecules inside a substance cool down, they lose energy and move around less. The movements of the molecules within a substance are what determine that substance’s state. In other words, when we heat a substance up or cool it down, we give energy to the molecules in that substance or take energy away from them. These changes to the energy and movement of the molecules in a substance change the state of matter that substance is in.
Let’s use the diagram above to consider these changes in water. When water molecules are cold and don’t have very much energy, they barely move at all. At this low level of energy, the water molecules form a highly organized repeating pattern called a crystal lattice. Magnetic interactions between the atoms of the water molecules called hydrogen bonds make it even more difficult for the molecules to move away from each other. When water is cold enough that its molecules can’t move, it is in its solid state, which we call ice. But if we add energy to the molecules in an ice crystal by heating it up, the ice begins to melt into liquid water.
In a liquid, molecules have more energy than they do in their solid state. In liquid water, water molecules have enough energy to temporarily break the magnetic hydrogen bonds they share with their neighboring molecules, but not enough energy to escape completely. So, the molecules begin to move around and slide past each other, but they stay close together. Because the molecules in a liquid squeeze close together, they are more dense than their solid counterparts. In other words, the same amount of molecules take up less space as a liquid than they do as a solid. Don’t believe me? Maybe it’s time we take a break for a little experiment.
|
To prove that water molecules take up more space in their solid forms than in their liquid form, we’re going to conduct an experiment. Find a clear container that you can fill with water or snow (this works best with a measuring cup, or something you can mark with dry-erase markers or masking tape). Fill the container with snow, and use the measuring lines, marker, or tape to guess how high the liquid water will fill the container after the snow has melted. Hint: it’s gonna be lower. Leave your container for several hours, and see what happens! If you’re feeling impatient, you can microwave your container for 10-15 second intervals until the snow has melted. But be careful not to microwave the snow for too long, or your water could boil into gas! If there isn’t any snow on the ground where you are when you do this activity, you can do the same experiment in reverse. Fill your container about half-way up with water, and then guess how high the ice will be in the container when it freezes. Put your container into a freezer, and check on it a day later to see how close your guess was! |
When water is cold, its molecules don’t have much energy, and they form a solid ice crystal. But when we heat that ice up, the molecules speed up enough to begin moving around, and the ice melts into liquid water. If we give our liquid water molecules even more energy by heating them up more, they become excited enough that they can completely break the magnetic attraction that they have with their neighboring water molecules. When this happens, individual molecules fly away from each other, and liquid water evaporates into water vapor, the gas form of water. The molecules in a gas aren’t connected to each other at all. They sometimes bump into each other as they fly around, but if they have enough energy they just bounce off of each other instead of sticking together.
Now we know why matter changes forms as it changes temperature. When the molecules in a substance heat up, they acquire more energy and begin to move around, changing the properties of the substance they form. This process also works in reverse. If a gas cools down enough, its molecules lose energy and may condense into a liquid. Let’s think about this with water. If you take a hot shower, some of the hot water from the shower evaporates into water vapor, or steam. But when your shower is done, water vapor from the shower that lands on cold surfaces in your bathroom turns back into a liquid. This is why taking a hot shower fogs up your bathroom mirrors!
Liquids can also change back into solids. If you put a glass of water into your freezer like you might have done for our experiment, the liquid water molecules will lose energy while they cool down. When they cool down enough, the magnetic forces between the water molecules will become stronger than the energy that makes the molecules move around. When that happens, the molecules will freeze into a crystal lattice, turning the liquid water into ice.
Snow Formation
By now we’ve answered some of our questions – we know how and why water changes forms as its temperature changes. When water molecules gain or lose energy as heat, they move differently, and these different interactions result in different states of matter. But we still haven’t answered one of our first questions. If we put a glass of water into our freezer, it turns into a solid chunk of ice, not a glass full of snow. So how does snow form? Now that we’ve learned more about the states of matter, we’re closer to understanding the next piece of our puzzle: clouds.
As we talked about before, when liquid water heats up, it turns into water vapor. As vapor, there is water in the air all around us! Tiny molecules of water fly invisibly through the air even in very dry places like Utah. Many of these molecules fly high up into the earth’s atmosphere where temperatures are lower than they are near the surface.
Can you guess what happens when molecules of water vapor reach these high, cold altitudes? If you guessed that they turn into a liquid or a solid, you are right! Like we learned earlier, molecules lose energy as they cool down. And when water molecules don’t have enough energy to break apart, they begin to stick together, making liquid and solid water.
When particles of water begin to condense into liquids and freeze into solids high in the air, they are so tiny and light that they float! These tiny particles float in the air, but they are large enough to bend and reflect sunlight, which makes them visible to us. In huge groups, these floating particles of water and ice make clouds! Clouds are made of countless tiny particles of solid and liquid water, floating in the air.
As particles of ice and water float around within a cloud, they occasionally collide. When they do, they sometimes connect, forming larger droplets of water or larger crystals of ice. When enough water droplets connect inside of a cloud, they become heavy enough that they can no longer float, and then they fall from the sky as drops of rain. But we aren’t talking about rain. If the air is even colder and the energy of the molecules within the cloud is even lower, tiny ice crystals are more common than water droplets. These crystals can also collide and connect in the clouds, and when they become heavy enough that they can’t float in the air, they slowly flutter to the ground as – you guessed it – snow.
Now we understand where snow comes from and why we can’t make it in a cup in our freezer. Snow forms from tiny particles of water vapor that soar high into the atmosphere and then freeze and collect into the crystals we know as snowflakes. When we freeze a relatively large amount of water in our freezer, that water instead turns into a solid block of ice instead of small snow crystals.
Snowflakes
Because of the ordered way that water molecules align when they freeze, all snowflakes have some features of their structure in common. Every snowflake is unique, but all snowflakes have six symmetrical arms! In other words, the six arms of a snowflake always look very similar.
However, no two snowflakes look exactly alike. Because every snowflake traces a unique path from the clouds to the ground, they encounter different temperatures and atmospheric conditions as they fall. Crystals grow as the snowflake falls through cold patches of atmosphere, and melt as they fall through warmer patches. Other factors, including humidity, also affect the formation of a snowflake’s crystals.
So, all snowflakes have six nearly identical arms because of the way water molecules align when they freeze. But since every snowflake takes a totally unique journey through the sky, no two snowflakes look the same.
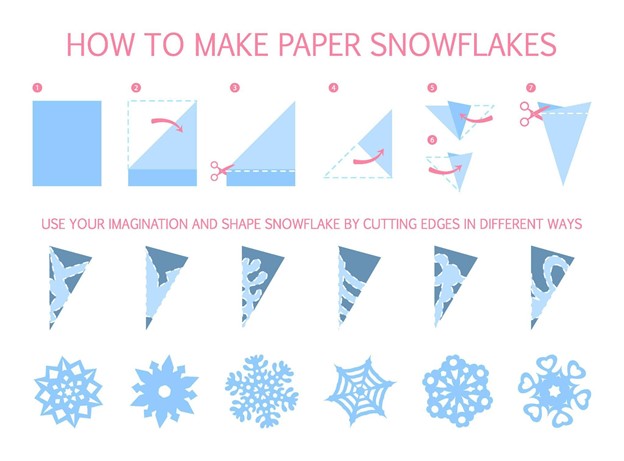
Let’s explore different snowflake shapes by making some model snowflakes of our own! You’ll need a piece of paper and some scissors to make these decorative snowflakes. Follow the instructions below and experiment with different patterns to make unique paper snowflakes of your own. Remember to recycle your paper snowflakes when you are done with them instead of throwing them away!
Conclusion: Snow is Weird, Cool, and Delicious
Let’s reflect on what we learned! The world as we know it is made up of matter, and some of the smallest building blocks of matter are atoms. Different kinds of atoms combine to make molecules, and those molecules make up everything we interact with in the world.
Matter can exist in different states depending on their temperature. Relatively cold matter exists as a solid. In this state, the molecules align in a rigid order. Warmer matter exists as a liquid. Molecules in a liquid are closely packed, but they slide around each other, making liquids more dense than solids. When matter is heated even more, it exists as a gas. Molecules in a gas move quickly and don’t often touch each other.
When liquid water heats up, it turns into water vapor, the gaseous form of water. That water vapor gradually drifts into the upper atmosphere, where it cools and condenses into drops of liquid water, or freezes into tiny ice crystals. Huge groups of these water droplets and crystals form clouds, but when they become heavy enough, they fall to the ground as rain or snow.
As snowflakes fall, their unique path through the atmosphere causes them to form into unique six-armed crystals. Look at you! You’ve learned so much about snow – down to its microscopic details! Thank you for joining us this month on a snow science journey. I think it’s time to celebrate – with a little more snow. For our last activity this month, we’re gonna learn how to make fresh snow into ice cream! I hope you enjoy the treat, and while you scoop some snow up to make into ice cream, imagine the journey that snow might have taken to end up as your dessert! We’ll see you next month on Trails. Online.
Fresh Snow Ice Cream
Prep Time: 5-10 Minutes
Yield: 2-4 Servings
Ingredients
- 1 Cup Milk (1 Can of Evaporated Milk or Sweetened Condensed Milk can also be used)
- ½ Cup Granulated Sugar
- 1 Teaspoon Vanilla Extract
- 1 Pinch Salt
- 8-12 Cups of Fresh, Clean Snow
- Any Ice Cream Toppings You Might Like
Instructions
- In a small bowl, whisk the milk, sugar, salt, and vanilla together until well combined.
- In a large bowl, add your 8-12 cups of snow. Make sure the snow you use is clean! Drizzle your milk mixture over the snow and stir well to combine. Your snow ice cream will not be as thick as store-bought ice cream, but it shouldn’t be runny.
- Add your favorite ice cream toppings and eat your snowy treat before it melts!

